Junhua Implantable AKSOPEEK® Self-Interpretation

After 20 years of rapid development, domestic orthopedics has gradually broken the monopoly of foreign products, but in terms of basic biomedical materials, some materials in China are still stuck.
Medical implant grade PEEK (polyetheretherketone) is such a material. In the past few decades, implant grade PEEK materials have been monopolized by three manufacturers in the world, so that we have to accept high-priced raw materials from abroad, and even some foreign brands of materials are not sold to the Chinese market. It is not possible to buy them at high prices, let alone centralized procurement with volume and price reduction.

Breaking the monopoly of foreign medical implant-grade materials has always been the goal and dream of domestic enterprises. However, why have domestic enterprises found it difficult to break through the foreign blockade for so many years? After long-term research and summary, there are four major difficulties:
1. Difficulty in updating production equipment and environment
Currently, there are about 5 domestic manufacturers with industrial-grade PEEK polymerization preparation capabilities, and almost all of them have formed mature industrial-grade PEEK polymerization processes and equipment. Due to the early development of these PEEK companies, it is difficult to meet the production requirements of implant-grade PEEK in terms of production environment layout, production equipment and related configurations.
Most of the domestic PEEK raw material suppliers do not have the qualifications for medical device production, and the relevant requirements and standards for implant-grade production lines are not clear. At the same time, chemical companies are very cumbersome to change equipment and production lines, and have great financial pressure. It takes a long time to explore and establish new production lines.

2. The purification process of implantable PEEK is difficult
From the product point of view, there is little difference in performance between industrial PEEK and implantable PEEK. The difference mainly lies in the purity of the material. To ensure the biocompatibility of PEEK materials for long-term use in the human body, it is necessary to ensure that the content of some trace chemical elements and heavy metal ions does not exceed the standard. At present, almost all PEEK produced by domestic enterprises have the problem of excessive content of trace elements and metal ions.
At present, this problem can only be dealt with in the process of polymerization and refining, and it is difficult to remove it even with high-pressure melt filtration in the later granulation. To solve this problem, PEEK manufacturers need to actively work with domestic equipment manufacturers to develop equipment that meets the requirements of implantable PEEK refining and purification, and study new purification processes. Only by making breakthroughs in both equipment and process can we ensure the effective removal of metal ions and trace chemicals during purification.
3. Difficulty in material and process compliance
Making a product is only the first step, and complying with YY/T 0660-2008 and 16886 related implant materials is only a basic requirement. As a domestic head implant-grade PEEK material manufacturer, when submitting product registration, it will definitely be strictly reviewed by the reviewer. The Food and Drug Administration will even conduct a flight inspection of the entire production process. The production company must at least have key process flows such as polymerization, refining, granulation, and extrusion.
How to show the reviewer the stability and reliability of the process throughout the production process, and how to show the traceability of the entire production and processing and manufacturing process, these are all very important and difficult points.
4. Acceptance and recognition still requires time
It will take a process for government regulatory agencies, medical device companies and hospital doctors to accept and recognize domestic materials.

As one of the earliest domestic companies engaged in the research, development, production and manufacturing of PEEK materials, Jiangsu Junhua pays great attention to the application of PEEK in the medical industry and has been committed to the research, development and production of medical implant-grade PEEK materials.
After 16 years of production and technology accumulation, the company started from the source of polymerization and established Shandong Junhao High Performance Polymer Co., Ltd. to polymerize implantable PEEK raw materials. In accordance with the production standards of medical implant materials, a GMP refining production workshop was established, and professional refining and purification equipment for implantable AKSOPEEK® polymerization was developed, which effectively solved the problem of excessive heavy metal ion content in implantable PEEK materials.

In order to further effectively realize full-process monitoring and traceability, the company selected the DCS central control system, which can effectively realize the control and detection of the main parameters inside the equipment during the production process, ensure that the production process is fully controllable, and the production data can be archived separately for subsequent traceability.

The company pilot-produced autoclave-implant-grade PEEK materials in 2020. In the same year, third-party tests were conducted on various biological aspects of the materials in accordance with the ISO10993 test standard, and all test results met the requirements.
In 2021, the first batch of mass-produced implant-grade AKSOPEEK® materials will be sent for biological and physical and chemical performance inspections in accordance with the YY/T0660-2008 medical industry standard. It is expected that all inspections will be completed around September 2022, and the master file filing with the State Pharmacy is planned to be completed in the same year.
Jiangsu Junhua Special Plastics uses an ICP metal chromatograph to detect metal ions, and its lead ion content is extremely low, less than 0.002%.
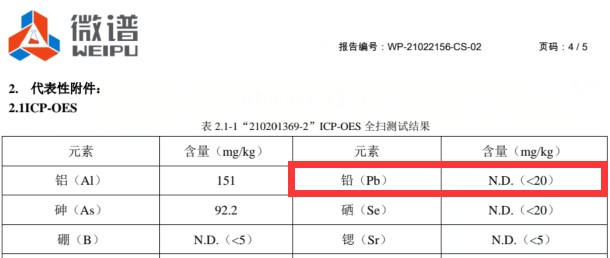
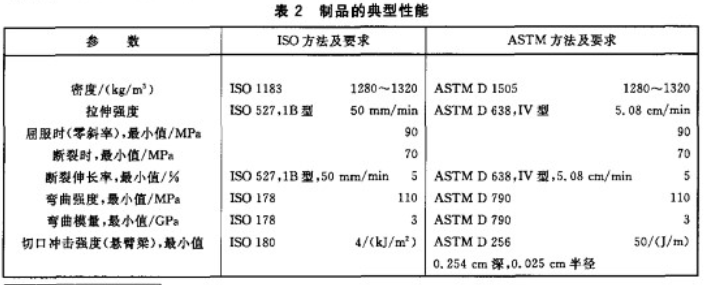
In addition to the basic requirements of implantable grade, the focus of customers is still on the mechanical properties of PEEK products. The quality of mechanical properties is related to whether patients can use it for a long time.
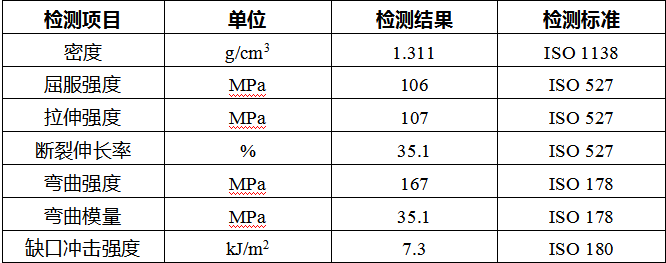
In response to the requirements on mechanical properties, Jiangsu Junhua Special Plastics has developed high-toughness AKSOPEEK®, which guarantees the patient’s postoperative recovery with performance far exceeding the standards.
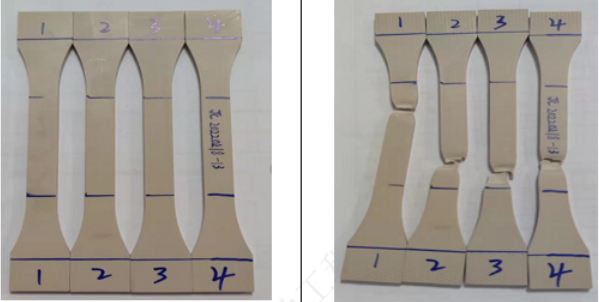
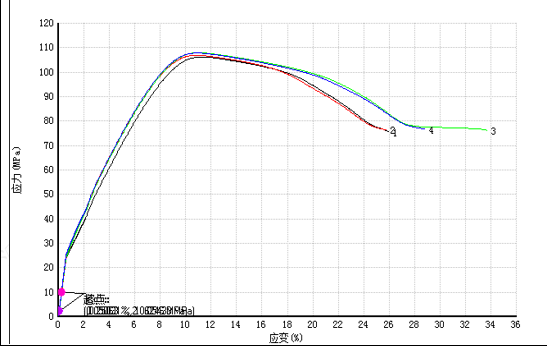
In addition, when we use implant-grade PEEK, there are also relevant medical industry testing specifications for certain specific uses, the most representative of which is the intervertebral fusion device.
Adopt YY/T 0959 (ASTM F2077) Spinal implant intervertebral fusion device mechanical properties test method test standard, under a load of 2000/4000N, a frequency of 5Hz, and a test number of 5 million times, the AKSOPEEK® Natural intervertebral fusion device (H is 6.3mm) was subjected to compression fatigue testing, and no failure due to fatigue occurred. Static tests before and after fatigue also did not show any strength reduction, and this model of fusion device will only show compression yield at 12000N.
